In the world of fluid mechanics, the classification of fluids into Newtonian and non-Newtonian Fluids plays an important role in understanding their behavior and applications. While the terms may sound complex, this article will break down the fundamentals, characteristics, and real-world applications of these Newtonian and non-Newtonian fluids with examples.
Newtonian and Non-Newtonian Fluids
Introduction
Fluids are all around us, from the water we drink to the air we breathe. Understanding how fluids behave is crucial in various fields, from engineering to medicine. One of the fundamental classifications of fluids in fluid mechanics is Newtonian and Non-Newtonian Fluids.
Newton’s Law of Viscosity
First, understand the term viscosity of fluid.
What is viscosity?
Viscosity refers to a fluid’s resistance to flow. In simpler terms, it measures how easily a liquid or gas can move through space. High-viscosity fluids are thick and flow slowly, while low-viscosity fluids are thin and flow quickly. The concept of viscosity is crucial in everyday life, from the oil in your car engine to the syrup on your pancakes.
Newton’s Contribution
Sir Isaac Newton was not only a pioneer in mathematics but also in the study of physics and fluid mechanics. In the 17th century, he formulated Newton’s Law of Viscosity, which states that
“The force required to move one layer of fluid past another is directly proportional to the velocity gradient and the area of contact, while inversely proportional to the distance between the layers.“
The Mathematical Expression
![]()
Where:
![]() is the Shear Stress =
is the Shear Stress = ![]()
‘F’ is the force applied to the fluid layers.
‘A' is the area of contact between the fluid layers.![]() is the is the dynamic viscosity of the fluid.
is the is the dynamic viscosity of the fluid.(du/dy) represents the velocity gradient, or the change in velocity with respect to distance.
Newtonian Fluids
Newtonian fluids, named after Sir Isaac Newton, exhibit a linear relationship between shear stress and shear rate (shear strain). In simpler terms, the force required to move a Newtonian fluid is directly proportional to the speed at which it is being moved. From this, we can simply say fluids that obey Newton’s law of viscosity are Newtonian fluids. This is true for all gases and most pure liquids.
Viscosity in Newtonian Fluids
Viscosity, or the resistance to flow, is a crucial parameter in Newtonian fluids. It remains constant, regardless of the shear rate applied. This predictable behavior makes Newtonian fluids ideal for various applications, including hydraulic systems and automotive lubricants.
Examples of Newtonian Fluids
All gases and liquids are Newtonian fluids like kerosene, alcohol, glycerin, benzene, hexane, ether, etc. Sugar and salts dissolved in water also behave like Newtonian fluids.
The relationships between the shear stress and shear rate
The figure below shows curves of shear stress against the rate of shear at constant temperature and pressure.
The simplest behavior is that shown by a curve, which is a straight line passing through the origin shows Newtonian fluids behavior.
The other curves shown in Figure represent the rheological behavior of liquids.
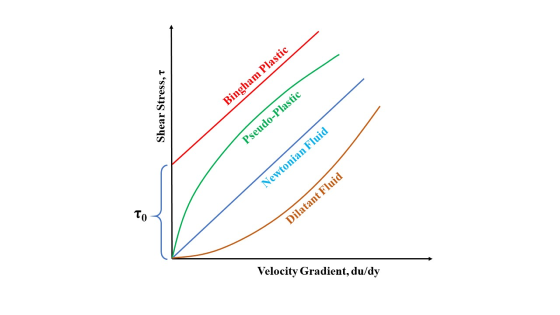
Non-Newtonian Fluids
Fluids for which the ratio of the shear stress to the shear rate is not constant but is considered as a function of the shear rate. Fluids that do not obey Newton’s law of viscosity are called non-Newtonian fluids.
Generally, liquids containing a second phase in suspension (solutions of finely divided solids and liquid solutions of large molecular weight materials) are non-Newtonian fluids.
Types of Non-Newtonian Fluids
There are three common types.
Bingham Plastics or Bingham Fluids
These fluids resist small shear stress but flow linearly under the action of larger shear stress.
Unless the threshold shear stress ![]() is exceeded, Bingham fluids do not deform or flow.
is exceeded, Bingham fluids do not deform or flow.
Representation of these fluids is as follows
![]()
![]()
Where ![]() is the yield stress (threshold shear stress) and
is the yield stress (threshold shear stress) and ![]() is commonly called the coefficient of rigidity.
is commonly called the coefficient of rigidity.
The topmost curve in the figure above shows this type of behavior.
Examples: Toothpaste, jellies, ketchup, shampoo, paints, sewage sludge, and some slurries.
Pseudoplastic Fluids
Pseudoplastic Fluids’ viscosity decreases with an increase in velocity gradient (shear rate).
The curve passes through the origin, is concave downward at low shears, and becomes nearly linear at high shears.
Pseudoplastic fluids are said to be shear-rate thinning fluids.
Examples: Paper pulp, Blood, Rubber latex, Solution of high molecular weight polymers, etc.
Dilatant Fluids :
Dilatant Fluids’ viscosity increases with an increase in velocity gradient.
The curve is concave upward at low shears and almost linear at high shears.
Dilatant fluids are said to be shear-rate thickening fluids.
Examples: Suspensions of starch in water, pulp in water, quicksand, and sand-filled emulsions.
The experimental curves for pseudoplastic and dilatant fluids can be represented by a power law, which is also called the Ostwald-de-Waele equation.
![]()
Where ![]() and
and ![]() are arbitrary constants.
are arbitrary constants.
Newtonian fluids : ![]()
Pseudoplastic fluids : ![]()
Dilatent fluids : ![]()
Pseudoplastics are said to be shear-rate-thinning and dilatant fluids are said to be shear-rate-thickening.
Time-Dependent Flow
None of the curves in the Figure above (shear stress vs. shear rate) depends on the history of the fluid, and a given sample of material shows the same behavior no matter how long the shearing stress has been applied.
Such is not the case for some non-Newtonian liquids, whose stress-vs.-rate-of-shear curves depend on how long the shear has been active.
Thixotropic fluids break down under continued shear and on mixing give lower shear stress for a given shear rate; that is, their apparent viscosity decreases with time.
Rheopectic substances behave in a reverse manner, and the shear stress increases with time, as does the apparent viscosity. The original structures and apparent viscosities are usually recovered on standing.
The rheological characteristics of fluids are summarized in the Table below
| Designation | Effect of increasing shear rate | Time dependent? |
| Pseudoplastic | Thins | No |
| Thixotropic | Thins | Yes |
| Newtonian | None | No |
| Dilatant | Thickens | No |
| Rheopectic | Thickens | Yes |
Shear-Thinning and Shear-Thickening
Non-Newtonian fluids can exhibit two primary behaviors: shear-thinning and shear-thickening. Shear-thinning fluids, like ketchup, become less viscous when agitated, making them easier to pour. On the other hand, shear-thickening fluids, such as a mixture of cornstarch and water, become more viscous under stress.
Key Difference between Newtonian and non-Newtonian Fluid
Viscosity Variation
The key difference between Newtonian and non-Newtonian fluid is how its viscosity behaves. While Newtonian fluids maintain a constant viscosity, non-Newtonian fluids can exhibit varying levels of viscosity.
Shear Rate (Shear Strain) Sensitivity
Non-Newtonian fluids are sensitive to changes in shear rate, which can lead to intriguing phenomena such as the Oobleck effect, where a mixture of cornstarch and water behaves as both a solid and a liquid depending on the applied force.
Newtonian vs. non-Newtonian: Which is Better?
Determining whether Newtonian or non-Newtonian behavior is preferable depends on the specific application. Both types of fluids have their strengths and weaknesses.
Selecting the right fluid for a particular task requires a deep understanding of fluid behavior and its implications.
Conclusion
The behavior of fluids can be classified into two categories: Newtonian and non-Newtonian fluids. Newtonian fluids follow Newton’s law of viscosity, while non-Newtonian fluids do not. Additionally, non-Newtonian fluids can be further classified into different types based on their behavior under shear stress, including Bingham Plastics, Pseudoplastic, and Dilatant Fluids. Understanding the characteristics of different types of fluids is crucial in various industries, including chemical, food, and pharmaceutical. By knowing the properties of different fluids, engineers, and scientists can design more efficient and effective processes.
Read Also:
FAQs
Examples of Newtonian fluids: All gases and liquids, such as kerosene, alcohol, glycerin, benzene, hexane, ether, etc.
Examples of Non-newtonian Fluids:
1. Bingham Plastics or Bingham Examples: Toothpaste, jellies, ketchup, shampoo, paints, sewage sludge, and some slurries.
2. Pseudoplastic Fluids Examples: Paper pulp, Blood, Rubber latex, Solution of high molecular weight polymers, etc.
3. Dilatant Fluids Examples: Suspensions of starch in water, pulp in water, quicksand, and sand-filled emulsions.
Non-Newtonian fluids are those fluids that do not follow Newton’s law of viscosity. The ratio of the shear stress to the shear rate is not constant but is considered a function of the shear rate.
The key difference depends on how their viscosity behaves. While Newtonian fluids maintain a constant viscosity, non-Newtonian fluids can exhibit varying levels of viscosity.
Newtonian fluid exhibits a linear relationship between shear stress and shear rate (shear strain). In simpler terms, the force required to move a Newtonian fluid is directly proportional to the speed at which it is being moved.
Fluids for which the ratio of the shear stress to the shear rate is not constant but is considered as a function of the shear rate. Fluids that do not obey Newton’s law of viscosity are called non-Newtonian fluids.
Read Also:

This is a great tip especially to those fresh to the blogosphere.
Short but very accurate info… Many thanks for sharing this one.
A must read article!
Thank You !
Thanks for your blog, nice to read. Do not stop.
Thank You !
Very nice post..!
Awesome stuff. Thanks a lot.
You mentioned it exceptionally well!