The fundamental laws of heat transfer for the three modes of heat transfer are as follows. Fourier law of heat transfer for Conduction. Newton’s Law of Cooling for Convection. Stefan Boltzmann’s law, Plank’s law, and Wines displacement law are the laws of Radiation mode in heat transfer. Before discussing these laws, let’s understand some basic things about heat transfer.
Definition of Heat Transfer
The heat transfer occurs when heat energy transfers from one object to another. This happens due to a temperature difference.
The temperature difference is the driving force for heat transfer.
It studies the rates of heat exchange. The exchange occurs between a hot source and a cold receiver.
The heat transfer takes place according to the Second Law of Thermodynamics:
“Two objects at different temperatures will transfer heat when in thermal contact. Heat moves from the hotter object to the colder object.”
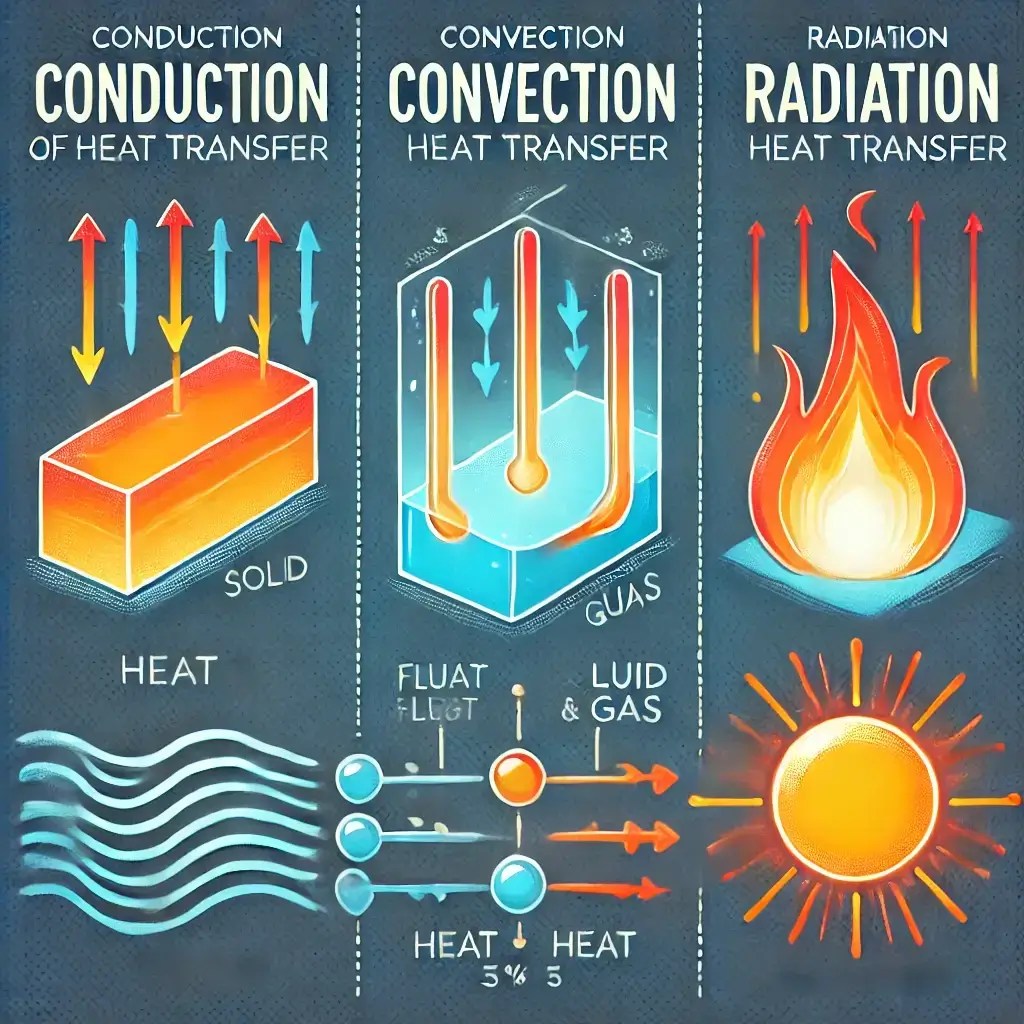
Three Modes of Heat Transfer
The following list illustrates the three modes of heat transfer
- Conduction
- Convection
- Radiation
Fundamentals of Conduction Heat Transfer
According to the laws of heat transfer, conduction heat transfer takes place when heat moves within an object. Heat transfers from one part of the object to another. It also happens when heat transfers from one object to another object. The objects must be in physical contact. There is no appreciable displacement of particles.
Heat transfer by conduction is mostly restricted to the flow of heat in solids. However, it occur in a stagnant liquid or, alternatively, in a gaseous medium.
Examples of conduction heat transfer:
- Heat flows through the brick wall of a furnace.
- The metal sheet of a boiler and the metal wall of a heat exchanger tube.
The basic law for conduction is Fourier’s law. Explained in detail below in the section on Laws of Heat Transfer.
Mechanism of Conduction
Conduction is the mode of heat transfer in which a material medium transporting the heat remains at rest. Heat conduction occurs by the migration of molecules. It is more effective when the molecules collide while vibrating around relatively fixed positions.
The molecules of a substance are always in a state of vibration. When the substance is heated at one of its locations, the molecules there receive energy. They begin to vibrate with larger amplitudes. As a result of this increased amplitude, they collide with neighboring molecules. In the process, they transfer part of their energy to these neighboring molecules. This process occurs repeatedly and thus results in heat flow from one molecule to another along the heat flow path.
Fundamentals of Convection Heat Transfer
Convection heat transfer occurs when there is the transfer of heat energy by the displacement of fluid. The fluid can be liquid or gas. This process happens from one place to another by mixing hot and cold portions of the fluid.
Convective heat transfer has two types
- Natural Convection or Free Convection
- Forced Convection
Natural Convection or Free Convection
In natural convection, fluid motion occurs because warmer and cooler fluids have different densities. This density difference arises from the temperature difference in the fluid mass.
As we know warmer fluid has a lower density than cooler fluid. Warmer fluid moves upward because of its low density. Cold fluid, having a higher density, automatically descends to take the place of the warmer fluid. In this way, it creates a circulating current due to density difference.
An example of Natural Convection is the heating of water in a vessel or a pot.
Forced Convection
Forced convection occurs when the fluid motion is produced by an external mechanical means. This includes a pump, a fan, a blower, or an agitator. It can also include an externally imposed pressure gradient.
Examples of Forced convection are
Heat flow to a fluid by being pumped through a heated pipe.
Mixing of hot and cold fluid in a vessel by means of an agitator.
The basic law for convection is Newton’s Law of Cooling. Explained in detail below in the section on Laws of Heat Transfer.
Fundamentals of Radiation Heat Transfer
Radiation heat transfer occurs through the transfer of heat energy from one object to another. This happens through space, without direct contact. The process is conducted by electromagnetic waves.
Radiation does not require any material or medium for heat transfer. Heat can be transferred in a vacuum also by the mode of radiation.
Radiation is the fastest mode of heat transfer.
Examples of heat transfer by radiation mode include the transfer of heat from the sun to the earth. Another example is the loss of heat from an unlagged steam pipe to the ambient air.
By the observation, we can conclude that heat transfer by conduction is slow, faster by convection, and fastest by radiation.
Basic Laws of Heat Transfer
Laws of Conduction Mode Heat Transfer
Fourier Law of Heat Conduction
The French scientist Joseph Fourier introduced the physical law governing heat transfer through a uniform material. This occurs by conduction whenever a temperature difference exists. The basic laws of heat transfer govern the conduction mode.
The rate of heat flow by conduction through a uniform (fixed) material is directly proportional to the area. This area is normal to the direction of the heat flow. It is also proportional to the temperature gradient in the direction of the heat flow.
Joseph Fourier
Mathematically Fourier law of heat transfer can be represented as follows.


Where,
‘Q’ is the rate of heat flow/transfer in watts (W).
‘A’ is the area normal to the direction of heat flow in m2
‘T’ is the temperature in K.
‘x’ is the distance measured normal to the surface in m
Temperature Gradient
Temperature gradient designated by (-dT/dx).
The negative sign is used in the equation because the temperature gradient. Which is negative (since with an increase in n there is a decrease in T, i.e., temperature decreases in the direction of heat flow). It makes the heat flow positive in the direction of temperature decrease.
Thermal Conductivity
The proportionality constant ‘k’ given in the Equation is called the thermal conductivity. It is a characteristic property of the material through which heat is flowing and varies with temperature. It is one of the so-called transport properties of the material (like viscosity, μ).
Thermal conductivity is a measure of the ability of a substance to conduct heat. The larger the value of k, the higher will be the amount of heat conducted by that substance.
Thermal conductivity is the quantity of heat passing through a material of unit thickness within a unit heat flow area. This occurs in a unit time with a unit temperature difference across the opposite faces of the material.
The unit of thermal conductivity is W/(m K) or J/(s m K).
Thermal conductivity depends upon the nature of the material and its temperature. The thermal conductivity of solids are higher than that of liquids. Liquids have higher thermal conductivity than gases.
In general, the thermal conductivity of gases ranges from 0.006 to 0.6 W/(m·K) while that of liquids ranges from 0.09 to 0.7 W/(m·K).
The thermal conductivity of metals varies from 2.3 to 420 W/(m·K). The materials having higher values of thermal conductivity are referred to as good conductors of heat.
The best conductor of heat is silver [k = 420 W/(m·K)]. Red copper follows with a conductivity of [k = 395 W/(m·K)]. Then comes gold [k = 302 W/(m·K)]. Finally, aluminum is [k = 210 W/(m·K)].
Heat/Thermal Insulators
The materials having low values of thermal conductivity [less than 0.20 W/(m·K)] are called and used as heat insulators to minimize the rate of heat flow. e.g. asbestos, glass wool, cork, etc.
Importance of thermal insulation
To prevent the excessive flow of heat to the system or surroundings, we use thermal insulation.
It is provided for the protection of personnel from skin damage through contact with very hot and very cold surfaces.
It provides a comfortable working environment.
Required properties of thermal insulator.
It should have very low thermal conductivity.
The thermal insulator should withstand in working temperature range of the system.
It should be sufficiently durable and have good mechanical strength.
It should be easy to apply, non-toxic, readily available, and inexpensive, and not be the constituent of fire hazards
The critical radius of thermal insulation
It is the outer radius of thermal insulation at which the rate of heat flow is maximum.
So, the addition of insulation up to a critical radius of the pipe will increase the heat loss from the pipe,
However, the addition of insulation thereafter will reduce heat loss from the pipe.
Convection Mode Basic Laws of Heat Transfer
Newton’s Law of Cooling
The rate of heat loss of a body is directly proportional to the temperature difference. This difference is between the body and its environment.

Where,
h = Heat transfer coefficient (W/m2K)
Heat Transfer Coefficient
It is the quantity of heat transferred in a unit of time through a unit area. This occurs at a temperature difference of one degree between the surface and surroundings.
The unit of heat transfer coefficient in the SI system is W/(m2K).
1/h is called thermal resistance.
Basic Laws of Radiation in Heat Transfer
Stefan–Boltzmann Law
The total energy emitted per unit area by a black body increases over time. This happens as its absolute temperature rises. This emission is directly proportional to the temperature raised to the fourth power.


Where,
Eb = Emissive power of the black body.
T = Temperature in K.
σ = Stefan-Boltzmann constant
= 5.67 × 10–8 W/(m2·K4)
Planck’s Law
Plank’s law is the relationship between the monochromatic emissive power of a black body, absolute temperature, and the corresponding wavelength.

Where,
Eb,λ= Monochromatic emissive power of the black body.
h = Planck’s constant.
k = Boltzmann constant.
c = Speed of light.
T = Absolute temperature.
λ = Wavelength of radiation.
Wien’s Displacement Law
The wavelength at which the maximum monochromatic emissive power is obtained is inversely proportional to absolute temperature.

Where,
λmax is in micrometers and T is in Kelvins, the value of constant C is equal to 2890.
Conclusion
In this article, we have discussed the laws of heat transfer, focusing on the principles of conduction, convection, and radiation. It explains how these laws govern the movement of heat energy and their applications in various real-world scenarios.
Modes of Heat Transfer and basic laws of heat transfer
Conduction:
- Heat transfer through direct contact between materials.
- Explained by the transfer of kinetic energy between adjacent particles.
- Common in solids like metals due to their closely packed atoms.
- Thermal conductivity determines the efficiency of conduction.
- Fourier’s law is important for the conduction mode of heat transfer.
Convection:
- Heat transfer through the movement of fluids (liquids or gases).
- Occurs through the circulation of fluid particles.
- Important in processes like natural convection (e.g., hot air rising) and forced convection (e.g., radiator heating).
- Applications include cooking, climate control, and geophysical phenomena.
- Newton’s law of cooling governs the convection mode of heat transfer.
Radiation:
- Heat transfer in the form of electromagnetic waves.
- Doesn’t require a medium; can occur in a vacuum.
- Governed by Stefan-Boltzmann’s Law (relating energy radiated to temperature).
- Essential in heat transfer from the sun to Earth and infrared thermography.
- Stefan–Boltzmann Law, Planck’s Law, Wien’s Displacement Law are the important laws of Radiation mode in heat transfer.
Applications
- Engineering: Used in designing heat exchangers, insulating materials, and cooling systems.
- Meteorology: Explains weather patterns and the Earth’s energy balance.
- Cooking: Understanding heat transfer aids in culinary techniques.
- Energy Efficiency: Helps optimize energy use in buildings and industrial processes.
- Medicine: Used in medical imaging, such as infrared cameras for diagnosing health issues.
-
What are the 3 principles of heat transfer?
Conduction: Transfer of heat through direct contact between materials, where heat flows from a higher-temperature region to a lower-temperature region.
Convection: Transfer of heat by the movement of fluids (liquids or gases), where the fluid circulates and carries heat away from the source.
Radiation: Transfer of heat through electromagnetic waves, without the need for a physical medium. Heat can be radiated through a vacuum, like the heat from the sun reaching Earth. -
What are the 4 types of heat transfer?
Conduction: Heat transfer through direct contact between molecules in a solid, liquid, or gas, driven by temperature differences.
Convection: Heat transfer through the bulk movement of fluids (liquids or gases), where warmer fluid rises and cooler fluid sinks, creating a heat flow.
Radiation: Heat transfer through electromagnetic waves, such as infrared radiation, which does not require a medium and can occur in a vacuum.
Advection: A specific form of convection where heat is transferred by the horizontal or vertical movement of a fluid, particularly significant in large-scale environmental processes like ocean currents and wind.

Comments are closed.